DFT study of the formation mechanism of anthraquinone from the reaction of NO2 and anthracene on NaCl clusters: the role of NaNO3†
Abstract
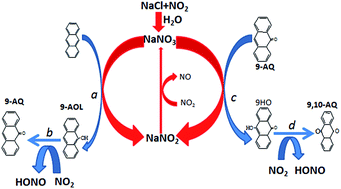
Polycyclic aromatic hydrocarbons (PAHs) and oxygenated-PAHs are globally worrisome air pollutants because of their highly direct-acting mutagenicity and carcinogenicity. The formation of oxygenated-PAHs is of crucial importance for the prevention of their atmospheric pollution successfully. In this paper, the formation mechanism of oxygenated-PAHs from the heterogeneous reaction of NO2 with anthracene on the surface of NaCl was studied by density functional theory (DFT) calculations. At first, the various adsorption configurations of NO2 and N2O4 on NaCl were investigated. The chemical conversion mechanisms among these configurations were also investigated. It is found that these structures can easily interconvert due to their low energy barriers. NaNO3 was found to be the main product of the reaction of NO2/N2O4 on NaCl. Then the oxidation mechanism of anthracene by NO2 on the NaCl surface showed that NaNO3 is able to oxidize anthracene and plays a catalytic role in the reaction process. This means that the formation of NaNO3 is very important to promote the formation of 9,10-anthraquinone from the heterogeneous reaction of NO2 with anthracene. Our calculations also showed that the introduction of water can greatly accelerate this reaction process.

 Please wait while we load your content...
Please wait while we load your content...