Material design of high-capacity Li-rich layered-oxide electrodes: Li2MnO3 and beyond†
Abstract
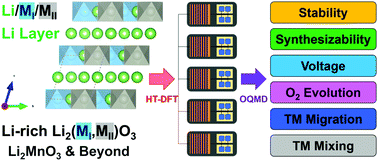
Lithium-ion batteries (LIBs) have been used widely in portable electronics, and hybrid-electric and all-electric vehicles for many years. However, there is a growing need to develop new cathode materials that will provide higher cell energy densities for advanced applications. Several candidates, including Li2MnO3-stabilized LiM′O2 (M′ = Mn/Ni/Co) structures, Li2Ru0.75Sn0.25O3 (i.e., 3Li2RuO3–Li2SnO3), and disordered Li2MoO3–LiCrO2 compounds can yield capacities exceeding 200 mA h g−1, alluding to the constructive role that Li2MO3 (M4+) end-member compounds play in the electrochemistry of these systems. Here, we catalog the family of Li2MO3 compounds as active cathodes or inactive stabilizing agents using high-throughput density functional theory (HT-DFT). With an exhaustive search based on design rules that include phase stability, cell potential, resistance to oxygen evolution, and metal migration, we predict a number of new Li2MIO3–Li2MIIO3 active/inactive electrode pairs, in which MI and MII are transition- or post-transition metal ions, that can be tested experimentally for high-energy-density LIBs.


 Please wait while we load your content...
Please wait while we load your content...