An efficient hydrogen evolution catalyst composed of palladium phosphorous sulphide (PdP∼0.33S∼1.67) and twin nanocrystal Zn0.5Cd0.5S solid solution with both homo- and hetero-junctions†
Abstract
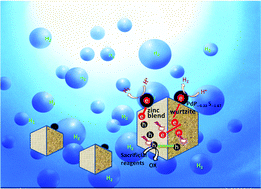
ZnxCd1−xS solid solutions have been successfully demonstrated as excellent visible light responsive catalysts for hydrogen evolution from water in the presence of sacrificial reagents. Especially Zn0.5Cd0.5S with a twin crystal structure is the best pristine sulphide catalyst ever reported so far due to the presence of the homo-junctions between the zinc blende (ZB) and wurtzite (WZ) segments, which improved the separation of the photon generated electron–hole pairs. However, a further improvement in the catalytic activities of the twin crystal Zn0.5Cd0.5S solid solution has never been reported. In the present work, palladium phosphorous sulphide (PdP∼0.33S∼1.67) was used for the first time as a co-catalyst and found that the photocatalytic H2 production rate and efficiency of the twin nanocrystal Zn0.5Cd0.5S solid solution in the presence of acidic or alkaline sacrificial reagents were significantly enhanced. The H2 production rates of Zn0.5Cd0.5S/PdP∼0.33S∼1.67 under visible light illumination (λ > 420 nm) in 0.75 M ascorbic acid (pH = 2.39) and 0.7 M Na2S/0.5 M Na2SO3 (pH = 13.86) aqueous solutions are measured to be 372.12 μmol h−1 mg−1 and 246.04 μmol h−1 mg−1, respectively, which are 67 and 5.8 times higher than those of pristine twinned Zn0.5Cd0.5S solid solution. Moreover, the H2 production rate of 372.12 μmol h−1 mg−1 is the highest value ever reported so far for sulphide photocatalysts in the presence of acidic sacrificial reagents. The examination on the morphology of the Zn0.5Cd0.5S/PdP∼0.33S∼1.67 hybrid catalyst using SEM, TEM and HRTEM techniques revealed the presence of hetero-junctions between the deposited PdP∼0.33S∼1.67 and Zn0.5Cd0.5S phases, as well as homo-junctions between the ZB and WZ phases in this hybrid catalyst. The synergistic effects of homo- and hetero-junctions on the separation of the generated electron–hole pairs are responsible for the significantly promoted catalytic activities of the hybrid catalyst. This result suggests that combination of both homo- and hetero-junctions in one catalyst is a promising strategy for improving the catalytic activities of sulphide catalysts for hydrogen evolution from water.

 Please wait while we load your content...
Please wait while we load your content...