Promising prospects for 2D d2–d4 M3C2 transition metal carbides (MXenes) in N2 capture and conversion into ammonia†
Abstract
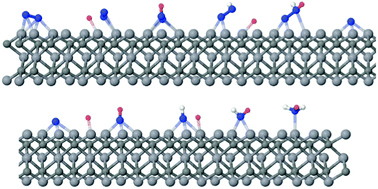
Density functional theory investigations of M3C2 transition metal carbides from the d2, d3, and d4 series suggest promising N2 capture behaviour, displaying spontaneous chemisorption energies that are larger than those for the capture of CO2 and H2O in d3 and d4 MXenes. The chemisorbed N2 becomes activated, promoting its catalytic conversion into NH3. The first proton–electron transfer is found to be the rate-determining step for the whole process, with an activation barrier of only 0.64 eV vs. SHE for V3C2.

 Please wait while we load your content...
Please wait while we load your content...