Theoretical capacity achieved in a LiMn0.5Fe0.4Mg0.1BO3 cathode by using topological disorder†
Abstract
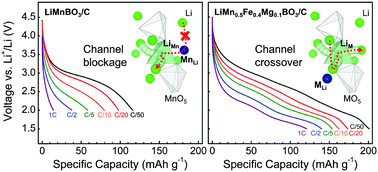
Simple borates are attractive cathodes for lithium-ion batteries due to two main reasons: covalently bonded anions provide operating stability through suppressed oxygen loss, and the borate group (BO3) possesses the highest theoretical specific capacity for one-electron polyanion systems. In this work, we demonstrate an electrochemically superior lithium borate (LiMn0.5Fe0.4Mg0.1BO3) that delivers a near theoretical capacity (98%) of 201 mA h g−1 at C/50, an improved rate capability of 120 mA h g−1 at 1 C, and good capacity retention. Using ab initio modeling, the superior Li intercalation activity is explained by both stabilization of the delithiated state and increased topological cation disorder, which counter-intuitively facilitates Li transport. Our results indicate that through engineering of defect chemistry, the basic mechanism can be modified from one-dimensional to three-dimensional conduction, thereby improving kinetics. Combined with the inherent stability of the borate group, the enhanced electrochemical properties should reinvigorate search in borate chemistry for new safe and high-energy cathode materials.

 Please wait while we load your content...
Please wait while we load your content...