Oxide enthalpy of formation and band gap energy as accurate descriptors of oxygen vacancy formation energetics†
Abstract
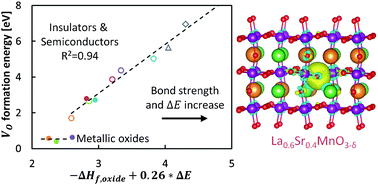
Despite the fundamental role oxygen vacancy formation energies play in a broad range of important energy applications, their relationships with the intrinsic bulk properties of solid oxides remain elusive. Our study of oxygen vacancy formation in La1−xSrxBO3 perovskites (B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cr, Mn, Fe, Co, and Ni) conducted using modern, electronic structure theory and solid-state defect models demonstrates that a combination of two fundamental and intrinsic materials properties, the oxide enthalpy of formation and the minimum band gap energy, accurately correlate with oxygen vacancy formation energies. The energy to form a single, neutral oxygen vacancy decreases with both the oxide enthalpy of formation and the band gap energy in agreement with the relation of the former to metal–oxygen bond strengths and of the latter to the energy of the oxygen vacancy electron density redistribution. These findings extend our understanding of the nature of oxygen vacancy formation in complex oxides and provide a fundamental method for predicting oxygen vacancy formation energies using purely intrinsic bulk properties.
Cr, Mn, Fe, Co, and Ni) conducted using modern, electronic structure theory and solid-state defect models demonstrates that a combination of two fundamental and intrinsic materials properties, the oxide enthalpy of formation and the minimum band gap energy, accurately correlate with oxygen vacancy formation energies. The energy to form a single, neutral oxygen vacancy decreases with both the oxide enthalpy of formation and the band gap energy in agreement with the relation of the former to metal–oxygen bond strengths and of the latter to the energy of the oxygen vacancy electron density redistribution. These findings extend our understanding of the nature of oxygen vacancy formation in complex oxides and provide a fundamental method for predicting oxygen vacancy formation energies using purely intrinsic bulk properties.

 Please wait while we load your content...
Please wait while we load your content...