An in situ carbon-grafted alkaline iron electrode for iron-based accumulators
Abstract
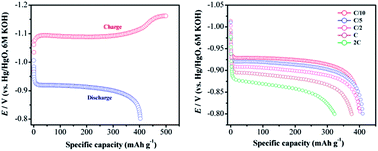
An in situ carbon-grafted alkaline iron electrode prepared from the active material obtained by decomposing the α-FeC2O4·2H2O–polyvinyl alcohol (PVA) composite at 600 °C in a vacuum is reported. The active material comprises a mixture of α-Fe and Fe3O4 with the former as the prominent component. A specific discharge capacity in excess of 400 mA h g−1 at a current density of 100 mA g−1 is obtained with a faradaic efficiency of 80% for the iron electrode made from carbon-grafted active material (CGAM). The enhanced performance of the alkaline iron electrode is attributed to the increased amount of metallic iron in the active material and its concomitant in situ carbon grafting.

 Please wait while we load your content...
Please wait while we load your content...