Long-term investigation of the photocatalytic hydrogen production on platinized TiO2: an isotopic study
Abstract
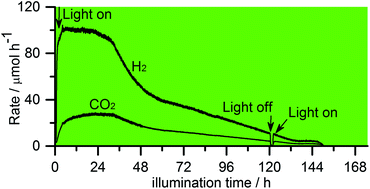
Long-term investigations of the photocatalytic hydrogen production on platinized TiO2 photocatalysts have been carried out employing different solutions of (deuterated) water and (deuterated) methanol. The results indicate that methanol acts as a sacrificial reagent, i.e., as an “electron donor” and that the amount of evolved molecular hydrogen is equivalent to the amount of H2 expected from the complete reforming of methanol or even less depending on the used photocatalyst. No evidence for photocatalytic water splitting is observed even in the presence of very low methanol concentrations, i.e., no molecular oxygen has been detected. Based upon the isotopic labelling studies it was confirmed that H2 is mainly produced by the reduction of protons originating from water.

 Please wait while we load your content...
Please wait while we load your content...