Proton relay and electron flow in the O–O single bond formation in water oxidation by the ruthenium blue dimer†
Abstract
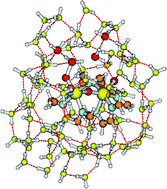
The first, key step of water oxidation catalysis by the ruthenium blue dimer transition metal complex has been studied via density functional methods and with extensive explicit solvation, starting from the oxidized catalytically active form of the dimer. This step is the rate-limiting O–O single bond formation. This reaction is found to involve several proton transfers through a proton relay chain, synergetically coupled to electron flow through the μ-oxo bridge of the dimer. The barrier for the O–O formation step is found to arise primarily from the surrounding aqueous solvent, suggesting that it might be substantially lowered in suitable environments. Some remarks are given concerning the following, penultimate step prior to the formation of dioxygen and the stable form of the dimer, in which it is suggested that another proton relay chain is at play.

 Please wait while we load your content...
Please wait while we load your content...