Carbon dioxide utilization with C–N bond formation: carbon dioxide capture and subsequent conversion
Abstract
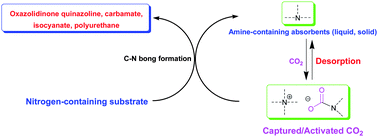
Carbon dioxide chemistry (in particular, capture and conversion) has attracted much attention from the scientific community due to global warming associated with positive carbon accumulation. The most widely used chemical absorption technique for carbon capture and storage/sequestration (CCS) would be essentially adopting amino-containing absorbents through formation of C–N bond in terms of mechanistic consideration. However, extensive energy input in desorption and compression process would be a crucial barrier to realize practical CCS. On the other hand, CO2 is very attractive as an environmentally friendly feedstock to replace the hazardous phosgene route for making commodity chemicals, fuels, and materials from a standpoint of green chemistry, whereas the reactions involving CO2 are commonly carried out at high pressure, which may not be economically suitable and also pose safety concerns. The challenge is to develop catalysts that are capable of activating CO2 under low pressure (preferably at 1 atm), and thus incorporating CO2 into organic molecules catalytically. We have proposed a carbon capture and utilization (CCU) strategy as an alternative approach to addressing the energy penalty problem in CCS. The essence of our strategy is to use captured CO2, also considered as the activated form of CO2, which could render this system suitable for accomplishing chemical transformation of CO2 under low pressure to avoid an additional desorption step. Indeed, CO2 could be activated through the formation of carbamate/alkyl carbonate with Lewis basic nitrogen species. In this review, we would like to discuss and update advances on CCU, particularly C–N bond formation with the production of oxazolidinones, quinazolines, carbamates, isocyanates and polyurethanes by using CO2 as C1 feedstock, and CO2 capture by amino-containing absorbents, including conventional aqueous solution of amine, chilled ammonia, amino-functionalized ionic liquids and solid absorbents such as amino-functionalized silica, carbon, polymers and resin, presumably leading to CO2's activation and thus subsequent conversion through C–N bond formation pathway.

 Please wait while we load your content...
Please wait while we load your content...