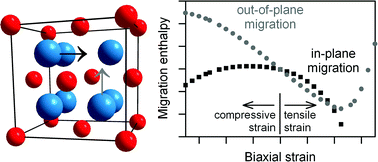
Static lattice simulation techniques were used to examine the effect of strain on oxygen-vacancy migration in the fluorite-structured oxygen-ion conducting electrolyte CeO2. Activation energies for vacancy migration, ΔEmig, were calculated as a function of isotropic and biaxial strain. In both cases, significant modification of the energetic barriers for oxygen-vacancy migration was found. Analysis of the data yields the activation volumes, ΔVmig, and activation enthalpies, ΔHmig. Simple comparisons based on the calculated data suggest that a biaxial, tensile strain of 4% may increase the in-plane conductivity at T = 500 K by close to four orders of magnitude. Enhancement of the oxygen-ion conductivity of an oxide heterostructure through space-charge effects is also discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...