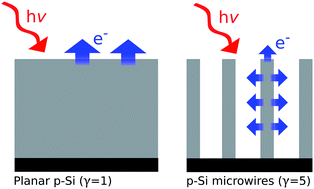
The dark electrocatalytic and light photocathodic hydrogen evolution properties of Ni, Ni–Mo alloys, and Pt on Si electrodes have been measured, to assess the viability of earth-abundant electrocatalysts for integrated, semiconductor coupled fuel formation. In the dark, the activities of these catalysts deposited on degenerately doped p+-Si electrodes increased in the order Ni < Ni–Mo ≤ Pt. Ni–Mo deposited on degenerately doped Si microwires exhibited activity that was very similar to that of Pt deposited by metal evaporation on planar Si electrodes. Under 100 mW cm−2 of Air Mass 1.5 solar simulation, the energy conversion efficiencies of p-type Si/catalyst photoelectrodes ranged from 0.2–1%, and increased in the order Ni ≈ Ni–Mo < Pt, due to somewhat lower photovoltages and photocurrents for p-Si/Ni–Mo relative to p-Si/Ni and p-Si/Pt photoelectrodes. Deposition of the catalysts onto microwire arrays resulted in higher apparent catalytic activities and similar photoelectrode efficiencies than were observed on planar p-Si photocathodes, despite lower light absorption by p-Si in the microwire structures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...