The pivotal role of electronics in preferred alkene over alkyne Ni–carboryne insertions and absolute regioselectivities†
Abstract
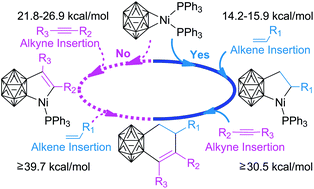
The in situ formation mechanisms of active Ni–carboryne species (COM1) and subsequent alkene/alkyne Ni–C bond insertion priorities, as well as relevant cycloaddition regioselectivities and kinetics, were investigated using the IDSCRF-B3LYP density functional theory (DFT) method, and all atoms were equitably treated at the DGDZVP level. The results reveal the o-carborane species to be energetically hedged into a four-step path (barrier heights 5.3, 19.7, 18.4 and 0.3 kcal mol−1, respectively) prior to being transferred into the active Ni–carboryne species (COM1) with the assistance of nBuLi and NiCl2(PPh3)2 at room temperature. In direct agreement with empirical trends, alkene insertion into Ni–C bonds on COM1 is exclusively favoured over the competing alkyne insertion. Electronic structure analyses of the corresponding transition structures showed that the preference of alkenes to alkynes is due to different bonding characteristics during this insertion process, namely, back donation for alkenes but donation for alkyne insertion, as evidenced by molecular graphics and NBO charge distributions. Subsequent alkyne additions (i.e. post alkene insertion) arise as the rate-determining step (RDS) for each of the five different reactions (a–e) explored. The solution free-energy barriers of these RDSs (30.5–38.5 kcal mol−1) were in quantitative agreement with their corresponding experimental yields, evidencing the reliability of the DFT results to reproduce chemical phenomena and energetic trends in real Ni-catalysed carboryne-alkene/alkyne cycloadditions.


 Please wait while we load your content...
Please wait while we load your content...