Synthesis, structures and luminescence properties of amine-bis(N-heterocyclic carbene) copper(i) and silver(i) complexes†
Abstract
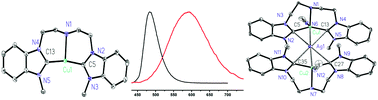
A series of Ag(I) and Cu(I) complexes [Ag3(L1)2][PF6]3 (8), [Ag3(L2)2][PF6]3 (9), [Cu(L1)][PF6] (10) and [Cu(L2)][PF6] (11) have been synthesized by reactions of the tridentate amine-bis(N-heterocyclic carbene) ligand precursors [H2L1][PF6]2 (6) and [H2L2][PF6]2 (7) with Ag2O and Cu2O, respectively. Complexes 10 and 11 can also be obtained by transmetalation of 8 and 9, respectively, with 3.0 equiv. of CuCl. A heterometallic Cu/Ag–NHC complex [Cu2Ag(L1)2(CH3CN)2][PF6]3 (12) is formed by the reaction of 8 with 2.0 equiv. of CuCl. All complexes have been characterized by NMR, electrospray ionization mass spectrometry (ESI-MS), and single-crystal X-ray diffraction studies. The luminescence properties of 10–12 in solution and the solid state have been studied. At room temperature, 10–12 exhibit evident luminescence in solution and the solid state. The emission wavelengths are found to be identical at 483 nm in CH3CN, but they are 484, 480 and 592 nm in the solid state for 10–12, respectively. These results suggest that 12 dissociates into two molecules of 10 and Ag(I) ions in solution. Complex 12 is the first luminescent heterometallic Cu/Ag–NHC complex.


 Please wait while we load your content...
Please wait while we load your content...
