A cation size effect on the framework structures in ABi2SeO3F5 (A = K and Rb): first examples of alkali metal bismuth selenite fluorides†
Abstract
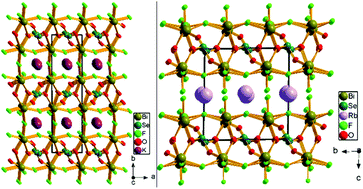
Two new centric alkali metal bismuth selenite fluorides, ABi2SeO3F5 (A = K and Rb), have been synthesized through a soft hydrothermal method. KBi2SeO3F5 crystallizes in an orthorhombic crystal system with the centric space group Pbcm possessing a three-dimensional (3D) network made up of SeO3 trigonal pyramids and asymmetric BiO3F5 polyhedra. As regards compound RbBi2SeO3F5, it crystallizes in a triclinic crystal system with a centric space group of P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , which contains alternant two-dimensional (2D) [Bi2SeO3F5]∞1− layers separated by Rb+ cations. In terms of structure, the differences between KBi2SeO3F5 and RbBi2SeO3F5 originate from the size of alkali metal cations, which has a great influence on the framework geometry and the dimensionalities of crystals. The UV-vis diffuse reflectance spectroscopy study of powder samples indicates that the band gaps of KBi2SeO3F5 and RbBi2SeO3F5 are approximately 4.08 and 4.18 eV, respectively, which are the largest ones among the known bismuth selenites owing to the existence of alkali metal cations as well as fluorine anions in crystals. Furthermore, theoretical calculations show that the optical absorption of two compounds is mainly attributed to the contribution of BiOxFy polyhedra and [SeO3]2− anionic groups.
, which contains alternant two-dimensional (2D) [Bi2SeO3F5]∞1− layers separated by Rb+ cations. In terms of structure, the differences between KBi2SeO3F5 and RbBi2SeO3F5 originate from the size of alkali metal cations, which has a great influence on the framework geometry and the dimensionalities of crystals. The UV-vis diffuse reflectance spectroscopy study of powder samples indicates that the band gaps of KBi2SeO3F5 and RbBi2SeO3F5 are approximately 4.08 and 4.18 eV, respectively, which are the largest ones among the known bismuth selenites owing to the existence of alkali metal cations as well as fluorine anions in crystals. Furthermore, theoretical calculations show that the optical absorption of two compounds is mainly attributed to the contribution of BiOxFy polyhedra and [SeO3]2− anionic groups.


 Please wait while we load your content...
Please wait while we load your content...