Alkene-alkyl interconversion: an experimental and computational study of the olefin insertion and β-hydride elimination processes†
Abstract
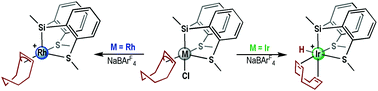
The preparation and characterization of various alkyl, allyl or alkene Rh(III) and Ir(III) complexes as well as studies on the intramolecular reactions leading to transformation of one into another are reported. The silyl-hydrido-Rh(III) complex {Rh(H)[SiMe(o-C6H4SMe)2](PPh3)}[BArF4], with a vacant coordination site, reacts with 1,5-cyclooctadiene (cod) leading to olefin insertion into the Rh–H bond and rearrangement to yield the 16e cyclooctenyl-Rh(III) complex {Rh(η3-cyclooctenyl)[SiMe(o-C6H4SMe)2]}[BArF4] (1). This compound can be also synthesized by reaction of the 18e chloride precursor {Rh(η3-cyclooctenyl)[SiMe(o-C6H4SMe)2]Cl} with NaBArF4. The reaction of the thioether-silane SiMeH(o-C6H4SMe)2 with [Rh(nbd)Cl]2 (nbd = norbornadiene) leads to {Rh(σ-ntyl)[SiMe(o-C6H4SMe)2]Cl} (ntyl = nortricyclyl) (2). The abstraction of chloride from this neutral 16e ntyl-Rh(III) complex with NaBArF4 results in the unusual isomerization of σ-nortricyclyl into σ,π-norbornenyl forming the 16e and cationic {Rh(σ,π-nbyl)[SiMe(o-C6H4SMe)2][BArF4] (nbyl = norbornenyl)} compound 3. Coordinatively saturated {Ir(η3-cyclooctenyl)[SiMe(o-C6H4SMe)2]Cl} (4) has been synthesized by the reaction of [Ir(cod)Cl]2 with SiMeH(o-C6H4SMe)2. The reaction of 4 with NaBArF4 led to the formation of the unsaturated and cationic Ir(III) compound {Ir(η3-cyclooctenyl)[SiMe(o-C6H4SMe)2]}[BArF4] (5). Compound 5 shows low stability in solution and undergoes successive β-hydride elimination and olefin insertion steps, which were elucidated by DFT calculations, to form 18e {Ir(H)[SiMe(o-C6H4SMe)2](η4-cod)}[BArF4] (6).


 Please wait while we load your content...
Please wait while we load your content...