A novel mechanism of heme degradation to biliverdin studied by QM/MM and QM calculations†
Abstract
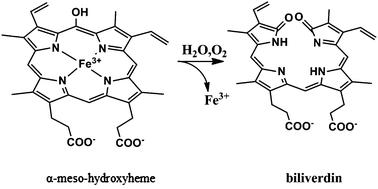
Heme degradation by heme oxygenase enzymes is important for maintaining iron homeostasis and prevention of oxidative stress. Previous studies have reported that heme degradation proceeds through three consecutive steps of O2 activation: the regiospecific self-hydroxylation of heme, the conversion of hydroxyheme to verdoheme and CO, and the cleavage of the verdoheme macrocycle to release biliverdin and free ferrous iron. Our results indicate that in the second step of heme degradation, not only verdoheme is generated but ring opening and biliverdin production also occur. We have performed QM-cluster and QM/MM calculations, which show that calculations with H2O as the axial ligand of Fe give the lowest barrier. In the QM-cluster calculation, the reaction is exothermic by −85 kcal mol−1 and the rate-limiting barrier is 5 kcal mol−1, whereas the corresponding QM/MM calculations give a slightly lower barrier of 3 kcal mol−1, owing to strong hydrogen bonds and the protein environment.


 Please wait while we load your content...
Please wait while we load your content...