Eu(iii) and Cm(iii) tetracarbonates – in the quest for the limiting species in solution†
Abstract
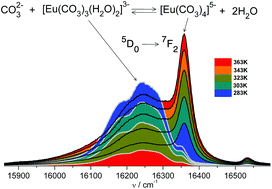
The structural and spectroscopic properties of the compounds [C(NH2)3]5[Gd:M(CO3)4(H2O)]·0.75H2O (1) and [C(NH2)3]5[Y:M(CO3)4]·2H2O (2) (M = Eu, Cm) were determined. The crystals contain differently hydrated tetracarbonate complexes, [M(CO3)4(H2O)]5− and [M(CO3)4]5−, which were used as structural and spectroscopic models of Eu(III) and Cm(III) tetracarbonate species in aqueous solutions. The luminescence spectra of the crystals were used to establish the stoichiometry and stability of the limiting species of the aqueous Eu(III) and Cm(III) carbonate systems at different temperatures and in a broad range of ionic strengths. By implementing this method together with the Pitzer approach used for the description of highly concentrated systems, it was possible to determine the thermodynamic functions of the reaction [Eu(CO3)3]3− + CO32− ⇆ [Eu(CO3)4]5− under standard conditions for the first time (ΔH° = 31.4 ± 2 kJ mol−1 and ΔS° = 82 ± 10 J mol−1 K−1). The proposed model for Eu(III) carbonates is consistent with the data recorded for the Cm(III)–carbonate systems. The presented results are important not only from the point of view of environmental issues, but also for the coordination chemistry of f-elements in general.


 Please wait while we load your content...
Please wait while we load your content...