Mg2+ incorporated Co-based MOF precursors for hierarchical CNT-containing porous carbons with ORR activity†
Abstract
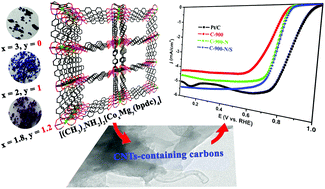
In this paper, we introduced light and abundant metal magnesium into a cobalt-based metal organic framework (Co-MOF, [(CH3)2NH2]2[Co3(bpdc)4]·5DMF·4CH3OH) (1, H2bpdc = 4,4′-biphenyldicarboxylic acid, DMF = N,N-dimethylformamide) as a heteroatom to synthesize Mg–Co bimetallic MOFs, namely [(CH3)2NH2]2[MgCo2(bpdc)4]·4DMF·5CH3OH (2) and [(CH3)2NH2]2[Mg1.2Co1.8(bpdc)4] 4DMF·4CH3OH·6H2O (3). Based on the formation of a rather low density framework after the introduction of the light Mg2+, such bimetallic MOFs exhibited higher gas adsorption abilities than the isostructural Co-based MOF 1. N2 adsorption measurements demonstrate that the BET surface area of 3 is 305.4 m2 g−1, exhibiting three times that of 1 (104.4 m2 g−1). Significantly, due to the introduction of the low-melting Mg2+, the Mg–Co MOFs could be further utilized as precursors for porous carbons only by calcination at a mild temperature of 600 °C which could exhibit a BET surface as high as 712.78 m2 g−1. Furthermore, after post-synthetic modification with a N/S heteroatom at 900 °C, the obtained hierarchical carbons exhibit superior activity in the oxygen reduction reaction (ORR) that is comparative to the commercial Pt/C catalyst. TEM results indicate that Co-embedded carbon nanotube (CNT)-containing hierarchically nanoporous carbons have been obtained. This study may offer a new avenue to prepare porous carbons utilizing Mg-containing bimetallic MOFs as sacrificial templates.


 Please wait while we load your content...
Please wait while we load your content...