Dehydrohalogenation of proton responsive complexes: versatile aggregation via pyrazolate pincer ligand arms†
Abstract
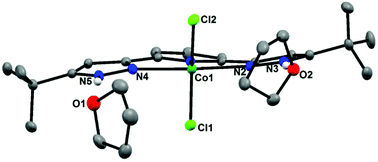
The behavior of the complex (H2L)CoCl2, where H2L is a bis-(pyrazol-3-yl)pyridine, towards Brønsted bases is studied, to evaluate peripheral NH deprotonation as a route to a dianionic pincer ligand on a d7 center. Deprotonation is found to also remove chloride from cobalt, and the decreased metal coordination number is then satisfied by bimolecular reaction of the newly formed peripheral deprotonated pyrazolate nitrogen, leading to Co2 units bridged by some of the pyrazolates, in the analogous species [Co2(L)(LH)]2(L) and [Co2(L)(HL)]2[Co(L)2], but also occasionally by chloride retention, in LiCo2L2Cl. Reacting LiCo2L2Cl with tBuNC, yields monomeric LCo(tBuNC)2, shown to be a 17 valence electron species. Use of excess LiN(SiMe3)2 in deprotonation of (H2L)CoCl2 leads to a product containing a Co[N(SiMe3)2]2 substructure, which illustrates opening of the Co2L2 dimer in response to an attacking nucleophile.


 Please wait while we load your content...
Please wait while we load your content...