Time dependent aggregation induced emission enhancement and the study of molecular packing in closely related azo-phenol BODIPY species†
Abstract
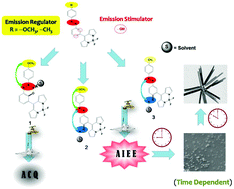
Fluorescent azo-phenol BODIPYs (1–3) have been obtained by the substituent (–OCH3/–CH3) directed synthesis of ortho (L1) and para (L2–L3) azo-phenol aldehydes. These display aggregation caused quenching (ACQ, 1) and aggregation induced emission enhancement (AIEE, 2 and 3) depending on the position of azo relative to the phenolic hydroxyl group. An intriguing time dependent morphological transition from nanospheres to ordered nanorods and subsequent emission changes in AIEE active azo-phenol BODIPYs have been successfully realized by time dependent fluorescence, scanning electron (SEM), transmission electron (TEM) and fluorescence optical microscopy (FOM) studies. The existence of one-dimensional (1D) nanorods as ultimate species in these compounds (2–3) has been supported by crystal packing patterns. Diverse aggregated forms and hierarchical nanostructures have been related to variable extents of fluorescence enhancement. The plausible charge transfer process and its role in AIEE have been supported by DFT studies.


 Please wait while we load your content...
Please wait while we load your content...