Structural diversity in the alkaline earth metal compounds of tetra and pentacyanocyclopentadienide†
Abstract
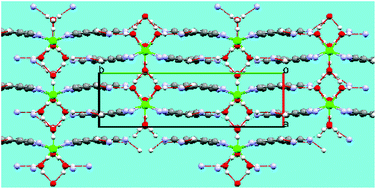
The reaction of the alkaline earth dihalides MCl2·nH2O (M = Mg, Ca, Sr, Ba) with the silver salts of cyanocyclopentadienides [C5(CN)4X]− (X = H, CN) yields the corresponding alkaline earth biscyclopentadienides. The pentacyanocyclopentadienides of Mg and Ca and the tetracyanocyclopentadienides of Ca, Sr and Ba were studied by X-ray diffraction. The Mg compound 1a′ shows only coordination by water molecules, but numerous hydrogen bridges between coordinated water molecules and pentacyanocyclopentadienide anions and further guest water molecules create a three-dimensional network. The structure of the calcium pentacyanocyclopentadienide 1b shows two inequivalent anions, one uncoordinated and one coordinating via three nitrile groups to three different metal ions. Ca2+ and [C5(CN)5]− ions together form a rhombohedral grid structure, in which the voids are filled by the uncoordinated [C5(CN)5]− ion and guest water molecules, all taking part in a hydrogen bond network too. The isostructural Ca and Sr tetracyanocyclopentadienides exhibit eightfold coordinated metal ions, in which both [C5(CN)4H]− anions coordinate to the metals by one cyano group each, and each anion bridging two metal ions. Thus, one-dimensional ribbons of {Ca[C5(CN)4H]4/2}x are formed, which are interconnected via hydrogen bridges from and to coordinated water molecules to build a three-dimensional network. The barium tetracyanocyclopentadienide contains a ninefold coordinated metal with the anion using three of its nitrile functions for coordination to three different metals. A complicated network is formed by four interpenetrating {Ba[C5(CN)4H]2} helices that are interconnected via the remaining third nitrile function, while the fourth nitrile group always remains uncoordinated. Although all coordinated water molecules are involved in hydrogen bridges, these are not contributing to the formation of the network.


 Please wait while we load your content...
Please wait while we load your content...