A modular approach to carbene-stabilized diphosphorus species†
Abstract
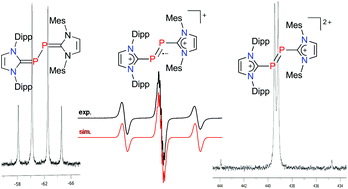
Heteroleptic N-heterocyclic dicarbene–diphosphorus species were prepared by reaction of the carbene–phosphinidene adduct (IPr)PSiMe3 (1, IPr = 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene) with the carbene–phosphorus trichloride adduct (IMes)PCl3 (2, IMes = 1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene), which furnished the dichloride [(IPr)PPCl(IMes)]Cl (3). Reduction of 3 with potassium graphite (KC8) afforded [(IPr)PP(IMes)] (4). The corresponding radical cation [(IPr)PP(IMes)]˙+ (5˙+) is isolated as [5]PF6 by reaction of 4 with ferrocenium hexafluorophosphate, whereas complexes containing the corresponding dication [(IPr)PP(IMes)]2+ (62+) can be isolated as the gallate and borate salts [6](GaCl4)2 and [6](BArF)2 by chloride abstraction from 3 with GaCl3 or sodium tetrakis[bis(3,5-trifluoromethyl)phenyl]borate (NaBArF), respectively. The asymmetric set of N-heterocyclic carbene ligands allows to establish 1JPP coupling constants of 249 Hz for 4 and 543 Hz for [6](GaCl4)2. Based on X-ray diffraction analyses, the molecular structures of 4, 5˙+ and 62+ reveal a consecutive shortening of the P–P bond lengths, in agreement with the presence of a phosphorus–phosphorus single bond in 4 and a double bond in 62+, which is best described as a dicationic diphosphene according to density functional theory (DFT) calculations.


 Please wait while we load your content...
Please wait while we load your content...