NMR effective radius of hydrogen in XIV group hydrides evaluated by NMR spectroscopy†
Abstract
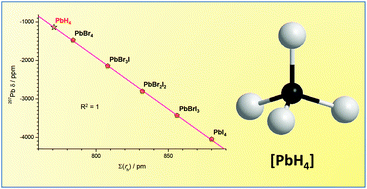
In the [ABrnIm] (A = C, Si, Ge, Sn; n + m = 4) compounds, with the heavier halides bonded to the central IV group elements, the experimental 13C, 29Si, 73Ge and 119Sn NMR chemical shifts of the central atoms were found to be strictly linearly proportional to the bonded halides ionic radii overall sum ∑(rh). Based on this, calibration lines relating the chemical shifts to ∑(rh) could be built for the considered subgroup of [ABrnIm] compounds. Using such calibration lines we could calculate the equivalent NMR radius, NMRrH–A, attributable to each of the bonded hydrogens in [AH4] species, according to the overall NMR shielding produced on the central A atom. Interestingly, the calculated NMRrH–A value resulted to be almost constant in all [AH4] examined systems (A = 13C, 29Si, 73Ge, 119Sn) with an average NMR![[r with combining macron]](https://www.rsc.org/images/entities/i_char_0072_0304.gif) H–A value equal to 194.6 ± 1.6 pm. Based on this approach, we could calculate the 207Pb NMR chemical shift of the unstable [PbH4] complex using the value of 192.7 pm calculated for NMRrH–Sn in the stable closest hydride [SnH4]. The obtained unprecedented NMR value is in accord with the 207Pb NMR chemical shift estimation, independently calculated for [PbH4] from the [SnH4] data, using the Pb/Sn chemical shift correlation defined in the Mitchell equation.
H–A value equal to 194.6 ± 1.6 pm. Based on this approach, we could calculate the 207Pb NMR chemical shift of the unstable [PbH4] complex using the value of 192.7 pm calculated for NMRrH–Sn in the stable closest hydride [SnH4]. The obtained unprecedented NMR value is in accord with the 207Pb NMR chemical shift estimation, independently calculated for [PbH4] from the [SnH4] data, using the Pb/Sn chemical shift correlation defined in the Mitchell equation.


 Please wait while we load your content...
Please wait while we load your content...