Novel half-sandwich iridium(iii) imino-pyridyl complexes showing remarkable in vitro anticancer activity†
Abstract
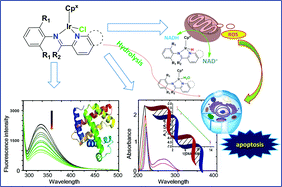
Seven novel half-sandwich IrIII cyclopentadienyl complexes, [(η5-Cpx)Ir(N^N)Cl]PF6, have been prepared and characterized, where Cpx is Cp* or the biphenyl derivative Cpxbiph (C5Me4C6H4C6H5), and the N^N-chelating ligands are imino-pyridyl Schiff-bases. The X-ray crystal structures of complexes 2A, 2B, and 3A have been determined. Excitingly, most of the complexes show potent antiproliferative activity towards A549 and HeLa cancer cells, except for Cp* complex 1A towards HeLa cells. Cpxbiph complex 2B displayed the highest potency, about 19 and 6 times more active than the clinically used drug cisplatin toward A549 and HeLa cells, respectively. These complexes undergo hydrolysis, and the kinetics data have been calculated. DNA binding has been studied by interaction with nucleobases 9-ethylguanine and 9-methyladenine, cleavage of plasmid DNA, and interaction with ctDNA. Interaction with DNA does not appear to be the major mechanism of action. Protein binding (bovine serum albumin, BSA) has been established by UV-Vis, fluorescence and synchronous spectroscopic studies. The stability of complex 2B in the presence of GSH was evaluated. The complexes catalytically convert coenzyme NADH to NAD+via hydride transfer. Cpxbiph complexes 2B and 4B induce cell apoptosis and arrest cell cycles at the S and G2/M phases towards A549 cancer cells and increase the reactive oxygen species dramatically, which appear to contribute to the remarkable anticancer activity.


 Please wait while we load your content...
Please wait while we load your content...