Rare earth metal–organic complexes constructed from hydroxyl and carboxyl modified arenesulfonate: syntheses, structure evolutions, and ultraviolet, visible and near-infrared luminescence†
Abstract
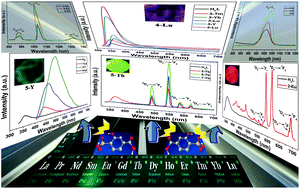
The reaction of 2-hydroxyl-4-carboxylbenzenesulfonic acid (H3L) and rare earth (RE) metal nitrates together with two N-heterocyclic ligands gives rise to the formation of 38 complexes, namely, [La(H2L)2(ox)0.5(H2O)4]n·2nH2O (1-La) (ox = oxalate), [RE2(H2L)2 (ox)(H2O)12]·2(H2L)·8H2O (2-RE) (RE = Nd, Sm, Eu, Gd, Tb, Dy), [RE(SO4)(H2O)7]·(H2L)·3H2O (3-RE) (RE = Ho, Er, Tm, Yb, Lu and Y), [RE(L)(H2O)3]n·nH2O (4-RE) (RE = Er, Tm, Yb and Lu), [RE(L)(2,2′-bipy)(H2O)]n (5-RE) (RE = La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho and Y, 2,2′-bipy = 2,2′-bipyridine), [RE(L)(1,10-phen)(H2O)]n (6-RE) (RE = La, Pr, Nd, Sm, Eu, 1,10-phen = 1,10-phenanthroline), and [RE(L′)(1,10-phen)2(H2O)]n (7-RE) (RE = Gd, Tb, Ho, Er, Yb and Lu, H3L′ = 2-hydroxy-3-nitro-4-carboxybenzenesulfonic acid), which have been characterized by elemental analysis, IR, TG, PL, powder and single-crystal X-ray diffraction. Complexes 1-La, 2-RE and 3-RE present zigzag chain, di- and mono-nuclear structures, in which H2L− acts as a counterion and monodentate and μ2-bridging monoanions. For the three species, light RE metal cations tend to induce the formation of oxalate while heavy RE metal cations tend to induce the formation of sulfate. Complexes 4-RE and 5-RE exhibit layer structures incorporating helical chains, in which the L3− trianion presents μ3 and μ4 coordination modes. Complexes 6-RE containing light RE metal cations show layer structures incorporating helical chains, while complexes 7-RE containing heavy RE metal cations have helical chain structures supported by the bridging of in situ generated L′3−. Remarkably, the in situ generated oxalates in 1-La and 2-RE, as well as the in situ generated L′3− in 7-RE, also play a crucial role in determining the structures of these complexes. Structure evolutions make these complexes present various luminescent emissions. Complexes 3-Tm, 3-Yb, 3-Lu, 3-Y and 4-Lu exhibit ultraviolet emissions from 354 to 370 nm. Complexes 1-La and 6-La present blue emissions at 442 and 463 nm. Complexes 2-Eu, 2-Tb, 5-Tb and 7-Tb exhibit characteristic red and green emissions while the complex 5-Y presents a green emission at 501 nm. Meanwhile, complexes 2-Nd, 3-Yb, 4-Yb, 5-Nd, 6-Nd, and 7-Yb show near-infrared (NIR) emissions. Moreover, 2-Eu, 2-Tb, 5-Tb, 7-Tb and 5-Y show longer luminescence lifetimes from 390.47 to 1211.57 μs.


 Please wait while we load your content...
Please wait while we load your content...