Iron(iii) complexes of 2-methyl-6-(pyrimidin-2-yl-hydrazonomethyl)-phenol as spin-crossover molecular materials†
Abstract
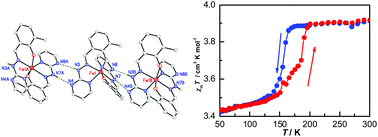
Four new mononuclear Fe(III) complexes 1–4 of 2-methyl-6-(pyrimidin-2-yl-hydrazonomethyl)-phenol (H2L) have been synthesized and characterized by magnetic susceptibility measurements and single-crystal X-ray diffraction analysis. Complexes 1–3 are ionic compounds with the formula [Fe(HL)2]X·nsolvent (X = ClO4− (1), BF4− (2) and Cl− (3)), while complex 4 ([Fe(HL)(L)]) is neutral. The central Fe(III) ion in complexes 1–4 is coordinated by four nitrogen and two oxygen atoms of two Schiff base ligands HL− or L2−, and the coordination geometries of FeIII are all distorted octahedral. Magnetic measurements indicate that complex 1 shows an abrupt spin transition with a thermal hysteresis of 32 K (T1/2↓ = 158 K and T1/2↑ = 190 K), and complex 4 exhibits a gradual incomplete spin transition. Complexes 2 and 3 are high spin with a room-temperature χmT value of 4.32 cm3 K mol−1 and 4.39 cm3 K mol−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...