A pyrene-modified cobalt salophen complex immobilized on multiwalled carbon nanotubes acting as a precursor for efficient electrocatalytic water oxidation†
Abstract
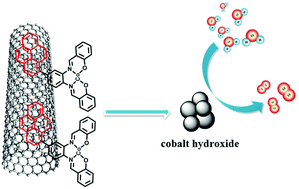
Immobilization of earth-abundant water oxidation catalysts (WOCs) on carbon supports to produce functional electrodes for electrochemical water splitting is a crucial approach for future clean energy production. Herein we report the non-covalent immobilization of a pyrene-bearing cobalt(II) Schiff base complex (2) on the surface of multiwalled carbon nanotubes (MWCNTs) to form a hybrid anode for electrocatalytic water oxidation. The 2/MWCNT anode displayed excellent catalytic activity and durability in neutral aqueous solution, and a catalytic current density of 1.0 mA cm−2 was achieved at 1.15 V vs. the normal hydrogen electrode (NHE), corresponding to a low overpotential of 330 mV. A Tafel slope of 96 mV per decade was obtained. The Faradaic efficiency of oxygen evolution was more than 90% by bulk electrolysis measurement. After bulk electrolysis, the hybrid anode characterization using X-ray photoelectron spectroscopy (XPS) confirmed that complex 2 decomposed to form heterogeneous cobalt hydroxides and the cobalt hydroxides should be true catalytic active species, which are responsible for electrocatalytic oxygen evolution.


 Please wait while we load your content...
Please wait while we load your content...