Noble-metal-free nickel phosphide modified CdS/C3N4 nanorods for dramatically enhanced photocatalytic hydrogen evolution under visible light irradiation†
Abstract
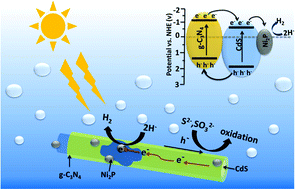
Photocatalytic hydrogen evolution is a promising technology in solving the global energy and environment issues. Therefore, it is urgent to develop highly efficient, nonprecious metal and stable photocatalysts. In this work, we synthesized a highly efficient Ni2P–CdS/g-C3N4 composite based on the concept of combining heterojunction engineering with co-catalyst modification. When employed as a photocatalyst for water splitting, the obtained best composite (5% Ni2P–CdS/g-C3N4) displayed dramatically enhanced hydrogen evolution activity at the rate of 44 450 μmol h−1 g−1, which was about 27 times higher than that of pure CdS (1668 μmol h−1 g−1). The apparent quantum yield at 420 nm reaches 46.3%. The excellent photocatalytic activity and stability can be ascribed to the synergistic effect of the intimate contact between CdS and g-C3N4 and the surface co-catalyst modification. Specifically, the g-C3N4 coated on the CdS nanorods can effectively promote the electron−hole pair separation spatially and Ni2P can lower the overpotential of H+ reduction.


 Please wait while we load your content...
Please wait while we load your content...