Exploration of an easily synthesized fluorescent probe for detecting copper in aqueous samples†
Abstract
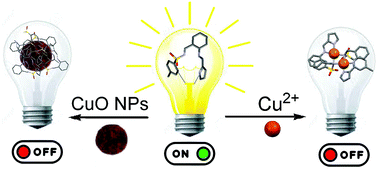
An easily synthesized fluorescent probe for detecting copper in aqueous samples, with a short response time and at neutral pH, has been investigated. Through the chelation of Cu2+ ions or by binding to CuO nanoparticles, the fluorescence emission of the 2-(aminomethyl)aniline derivative H2L is quenched by over 50%. Spectroscopic determination of the association constants of H2L with some metal ions showed that the ligand has a higher affinity toward Cu2+ than toward other d-block metal ions. The comparative bonding ability of the aniline-based fluorescent probe in d-block metal complexes was studied in solution by a combination of UV-Vis, 1H NMR and mass spectrometry analyses. Besides these, the bonding behavior has been investigated in the solid state by X-ray diffraction, FT-IR spectroscopy and elemental analysis. The crystal structures of Pd2(L)2 and Co(L)(HL)(H2O) were elucidated.


 Please wait while we load your content...
Please wait while we load your content...