Bistriazole-p-benzoquinone and its alkali salts: electrochemical behaviour in aqueous alkaline solutions†
Abstract
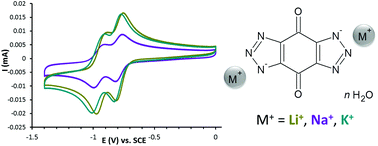
Quinones are well known as redox-active compounds. In this work bistriazole-p-benzoquinone was prepared and its electrochemical behaviour in aqueous alkaline solutions was studied by cyclic voltammetry. Two successive one-electron reduction steps were observed – the first step was reversible and the second quasireversible. Based on the nature of the alkali cation, the potential of the cathodic peak minimum and the anodic peak maximum was shifted towards positive direction as follows Li+ > Na+ > K+. In order to know more about the chemical structure of the alkali salts, the lithium, sodium and potassium salts of bistriazole-p-benzoquinone were crystallized and their structure could be revealed by single crystal X-ray analysis. Additionally, the thermal stability of the compounds was investigated by thermogravimetric analysis and variable temperature X-ray powder diffraction analysis.


 Please wait while we load your content...
Please wait while we load your content...