Simple ZnEt2 as a catalyst in carbodiimide hydroalkynylation: structural and mechanistic studies†
Abstract
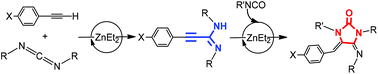
Expanding the possibilities of the use of simple and available ZnEt2 as a catalyst, the hydroalkynylation of carbodiimides with a variety of alkynes to obtain unsaturated substituted amidines is described in this work. Different stoichiometric studies allow proposing that amidinate complexes are intermediates in this catalytic process, produced by easy activation of the C–H bond of the alkyne and formation of alkynyl derivatives followed by a carbodiimide insertion step. Kinetics studies allowed the generation of a rate law for the hydroalkynylation of N,N′-diisopropylcarbodiimide with phenylacetylene which is second order in [carbodiimide], first order in [catalyst] and zero order in [alkyne], with a negligible PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH/PhC
CH/PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CD isotopic effect, consistent with a rate-determining state involving carbodiimide insertion. The hydroalkynylation reaction has been coupled with isocyanate (and isothiocyanate) insertion and intramolecular hydroamination to obtain imidazolidin-2-ones (or thione). The structures of different plausible intermediates have been determined by X-ray diffraction studies.
CD isotopic effect, consistent with a rate-determining state involving carbodiimide insertion. The hydroalkynylation reaction has been coupled with isocyanate (and isothiocyanate) insertion and intramolecular hydroamination to obtain imidazolidin-2-ones (or thione). The structures of different plausible intermediates have been determined by X-ray diffraction studies.


 Please wait while we load your content...
Please wait while we load your content...