Trimetallic PtAuNi alloy nanoparticles as an efficient electrocatalyst for the methanol electrooxidation reaction†
Abstract
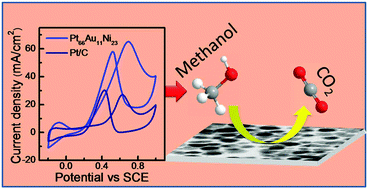
Platinum is an excellent electrocatalyst. However, the disadvantages of Pt as an electrocatalyst lie in its poor earth abundance, high cost, and poor stability due to surface poisoning. Thus it remains a challenge to find suitable alternative electrocatalysts and/or reduce the use of Pt. Herein, we report the solvothermal synthesis of bimetallic (PtAu and PtNi) and trimetallic (PtAuNi) alloy nanoparticles (NPs) with controlled percentages of individual metals. With an optimized Ni content, the trimetallic (Pt66Au11Ni23) alloy NPs show superior electrocatalytic activity (in terms of lower onset oxidation potential and higher mass activity) not only to bimetallic alloy NPs (PtAu and PtNi) but also to commercial Pt/C (20% Pt loading) for methanol electrooxidation (MEO). This enhanced electrocatalytic activity is due to the synergistic role of different metals in MEO catalysis. In particular, the catalytic activity is found to be controlled by the balance between the adsorption of methanol species on Pt and the removal of carbonaceous species from the catalyst surface by Au and Ni, as demonstrated here. The multimetallic alloy of optimal individual content thereby not only reduces the Pt content in the catalyst but also exhibits higher electrocatalytic activity than Pt/C for MEO that is desirable for fuel cell applications.


 Please wait while we load your content...
Please wait while we load your content...