Oxidant-free synthesis of benzimidazoles from alcohols and aromatic diamines catalysed by new Ru(ii)-PNS(O) pincer complexes†
Abstract
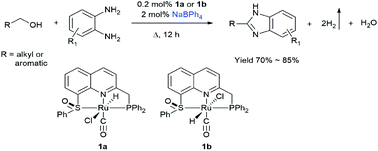
Benzimidazoles are chemically and pharmaceutically important, and an environmentally benign synthetic method based on acceptorless dehydrogenative condensation of primary alcohols and benzene-1,2-diamine is developed in this work. Three Ru(II) hydride complexes [RuHCl(CO)(PNS(O))] (containing two isomers 1a and 1b) and [RuHCl(CO)(PPh3)(SNCNHC)]PF6 (2) based on two new quinoline-based ligands 2-(diphenylphosphanylmethyl)-8-phenylsulfinylquinoline (PNS(O)) and 1-mesityl-3-(8-phenylthioquinolyl-2-methyl)-2-imidazole carbene (SNCNHC) are prepared and fully characterized. These complexes catalyse the condensation of benzyl alcohol and benzene-1,2-diamine to 2-phenylbenzimidazole with the liberation of H2, and the catalytic activity follows the order: 1a ≈ 1b > 2. When 0.2 mol% of 1a and 2 mol% of NaBPh4 were used, various 2-functionalized benzimidazoles were obtained in good yields (70–85%) and high turnover numbers (TONs ∼ 425). This homogeneous system does not need oxidants or stoichiometric strong bases (KOH or KOtBu, etc.) that are normally used in the reported homogeneous systems, and thus is a greener process.


 Please wait while we load your content...
Please wait while we load your content...