The influence of metal centres on the exchange interaction in heterometallic complexes with oxalate-bridged cations†
Abstract
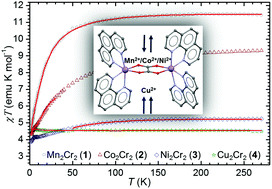
The reaction of bis(phenanthroline)metal(II) cations (M = Mn2+, Co2+, Ni2+, Cu2+ and Zn2+) with bis(oxalato)chromium(III) anions in a water/ethanol solution gives rise to a series of compounds with oxalate-bridged cations, [{M(phen)2}2(μ-C2O4)][Cr(phen)(C2O4)2]2·4H2O [Mn2Cr2 (1), Co2Cr2 (2), Ni2Cr2 (3), Cu2Cr2 (4) and Zn2Cr2 (5)]. Their structural analysis reveals that all the prepared compounds crystallize in the triclinic system, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , having similar unit cell parameters, molecular structures and crystal packing features. All metal centres in 1–5 are octahedrally coordinated: M2+ in homodinuclear cations are coordinated with two phen molecules and one bridging oxalate ligand; Cr3+ in anions is coordinated with one phen ligand and two bidentate oxalate groups. The copper atom in Cu2Cr2 (4) exhibits a Jahn–Teller-distorted octahedral coordination. Owing to the considerable number of pyridyl groups present in 1–5 (from phen ligands) the crystal packing of cations and anions is driven by stacking interactions appearing in offset-face-to-face (OFF) and edge-to-face (EF) orientations. The hydrogen bonds between the anions and water molecules of crystallization form 1D ladder-like motifs. In addition to the single crystal X-ray diffraction studies, the characterization of the new complexes was accomplished by means of IR and UV/Vis spectroscopy and magnetization measurements on a SQUID magnetometer. The temperature dependence of magnetic susceptibility reveals different magnetic super exchange interactions taking place in homodinuclear oxalate-bridged cations depending on the transition metal centre (Mn2+, Co2+, Ni2+ and Cu2+). Oxalate ligands mediate the ferromagnetic coupling of Cu2+ metal cations in Cu2Cr2 (4), whereas in Mn2Cr2 (1), Co2Cr2 (2) and Ni2Cr2 (3), antiferromagnetic interactions are observed between Mn2+, Co2+ and Ni2+ cations, respectively. Also, relatively large zero-field splitting parameters for Cr3+ ions (from mononuclear anions), D ≈ 1 cm−1, were observed.
, having similar unit cell parameters, molecular structures and crystal packing features. All metal centres in 1–5 are octahedrally coordinated: M2+ in homodinuclear cations are coordinated with two phen molecules and one bridging oxalate ligand; Cr3+ in anions is coordinated with one phen ligand and two bidentate oxalate groups. The copper atom in Cu2Cr2 (4) exhibits a Jahn–Teller-distorted octahedral coordination. Owing to the considerable number of pyridyl groups present in 1–5 (from phen ligands) the crystal packing of cations and anions is driven by stacking interactions appearing in offset-face-to-face (OFF) and edge-to-face (EF) orientations. The hydrogen bonds between the anions and water molecules of crystallization form 1D ladder-like motifs. In addition to the single crystal X-ray diffraction studies, the characterization of the new complexes was accomplished by means of IR and UV/Vis spectroscopy and magnetization measurements on a SQUID magnetometer. The temperature dependence of magnetic susceptibility reveals different magnetic super exchange interactions taking place in homodinuclear oxalate-bridged cations depending on the transition metal centre (Mn2+, Co2+, Ni2+ and Cu2+). Oxalate ligands mediate the ferromagnetic coupling of Cu2+ metal cations in Cu2Cr2 (4), whereas in Mn2Cr2 (1), Co2Cr2 (2) and Ni2Cr2 (3), antiferromagnetic interactions are observed between Mn2+, Co2+ and Ni2+ cations, respectively. Also, relatively large zero-field splitting parameters for Cr3+ ions (from mononuclear anions), D ≈ 1 cm−1, were observed.


 Please wait while we load your content...
Please wait while we load your content...