Abstract
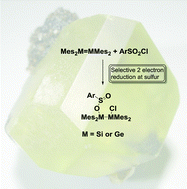
The addition of a variety of sulfones and a sulfoxide to ditetrelenes (a disilene and a digermene) was examined. The reaction of benzenesulfonyl chloride with tetramesityldisilene or tetramesityldigermene results in the formation of the 1,2-addition products, 2-chlorotetramesityldisilyl- or digermylbenzenesulfinate. The addition of p-toluenesulfonyl chloride to the disilene gave the analogous product, 2-chlorotetramesityldisilyl p-toluenesulfinate. In contrast, benzenesulfonyl fluoride, diphenyl and dimethyl sulfone did not react with either the disilene or the digermene. The unprecedented formation of the sulfinates reveals a selective 2-electron reduction of the sulfur centres using ditetrelenes. The addition reactions of sulfonyl compounds illustrates the potential of ditetrelenes to serve as reducing agents which react rapidly, at room temperature under mild conditions. The reaction of tetramesityldisilene with diphenyl sulfoxide resulted in the formation of tetramesityloxadisilirane and with benzene sulfonic acid resulted in the formation of 1,1,2,2-tetramesityldisilyl benzenesulfonate.
- This article is part of the themed collection: Philip Power at 65: an icon of organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...