Reduction of carbon dioxide with a superalkali
Abstract
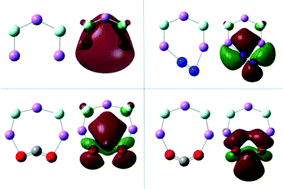
The ability of the superalkali Li3F2 to reduce (electron transfer) carbon dioxide (CO2) is presented. The CBS-QB3 composite method is employed to obtain reliable information on the geometries and energetics of the investigated species. Transition states and minima were located by scanning the potential energy surface for CO2 addition to the Li3F2 superalkali. The stability of Li3F2/CO2 is explained by high binding energy, charge flows, and the highest occupied molecular orbital–lowest unoccupied molecular orbital (HOMO–LUMO) gap. The selectivity of Li3F2 towards CO2 has also been computed by performing the same calculations for the most abundant atmospheric gas molecule N2. These results show a very small chemical affinity of Li3F2 for N2.


 Please wait while we load your content...
Please wait while we load your content...