Copper-coordination polymer-controlled Cu@N-rGO and CuO@C nanoparticle formation: reusable green catalyst for A3-coupling and nitroarene-reduction reactions†
Abstract
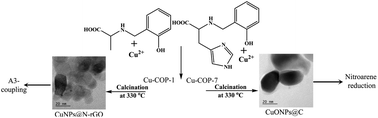
The intriguing structural properties of coordination polymers (COPs), together with the huge variety of metal ions and organic linkers to choose from, make COPs potential precursors for fabricating carbon-encapsulated metal and metal oxide nanoparticles (NPs). Herein, we have studied the role of the COP structural assembly, prepared through making subtle changes to the ligand structure, on the formation of NPs in a carbon matrix. Cu-COPs (Cu-COP-1–Cu-COP-7), generated using different amino acid-based reduced Schiff base phenolic chelating ligands, exhibited crystalline structures with differing structural organization in the solid state. Interestingly, the calcination of Cu-COP-1 and Cu-COP-5 at 330 °C produced pure CuNPs, whereas Cu-COP-2, Cu-COP-3, Cu-COP-4 and Cu-COP-7 gave CuONPs encapsulated by carbon matrix. The calcination of Cu-COP-6 produced both CuNPs and CuONPs together in the carbon matrix. The formation of CuNPs and CuONPs in the carbon matrices was unambiguously confirmed by PXRD and XPS studies. The sizes and morphologies of the Cu/CuONPs were analyzed using HR-TEM and FE-SEM. BET studies revealed higher surface areas with small pores for the CuNPs encapsulated by carbon and lower surface areas with higher porosity for the CuONP-carbon matrix. Raman spectra revealed the formation of a nitrogen-doped reduced graphene oxide (N-rGO) carbon matrix in CuNPs-1. The N-rGO coverage and high surface area with small pores provided CuNPs-1 with good stability in strong acid (10 M H2SO4). Importantly, the fabricated N-rGO-encapsulated CuNPs-1 and carbon-covered CuONPs-4 nanocomposites were used as green catalysts in solvent-free neat A3-coupling and nitroarene-reduction reactions, respectively. The products were confirmed using 1H-NMR spectra. The recovered CuNPs-1 and CuONPs-4 catalysts, after the completion of the reactions, also showed similar catalytic activity at up to five cycles, without significant loss of catalytic activity. Thus, the present studies demonstrate the influence of Cu-COP structural assembly on the formation of Cu/CuONPs as well as the carbon matrix, and open the possibility of fabricating functional nanomaterials from the vast number of available COPs with intriguing structural motifs.


 Please wait while we load your content...
Please wait while we load your content...