Lead(ii): Lewis acid and occasional base, as illustrated by its complex with 1,5-naphthalenedisulfonate and 5-methyl-1,10-phenanthroline†
Abstract
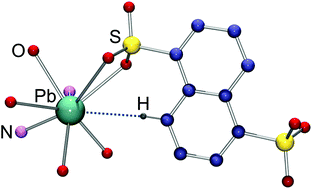
A crystal structure determination of the Pb(II) coordination polymer [Pb(Mephen)(1,5-nds)(H2O)]n provides not only evidence of the common action of Pb(II) as a Lewis acid but also clear proof of its ability, in the solid state at least, to act as a Lewis base. This action as a base is attributed to the presence of a valence shell lone pair and its identification here is further evidence for the occasional but not universally detectable influence of the lone pair on the metal ion stereochemistry.


 Please wait while we load your content...
Please wait while we load your content...