Metal-mediated reactions between dialkylcyanamides and acetamidoxime generate unusual (nitrosoguanidinate)nickel(ii) complexes†
Abstract
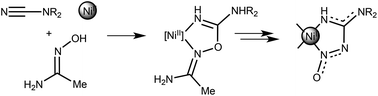
The nitrosoguanidinate complexes [Ni{NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NR2)NN(O)}2] (R2 = Me21, (CH2)4O 2, (CH2)43, (CH2)54, (Me)Ph 5, Ph26, (p-MeC6H4)27) were obtained in low-to-moderate (12–26%) yields but reproducible yields in an unexpected metal-mediated reaction in MeOH between the nickel salt NiCl2·2H2O, N,N-disubstituted cyanamides NCNR2, and the amidoxime MeC(
C(NR2)NN(O)}2] (R2 = Me21, (CH2)4O 2, (CH2)43, (CH2)54, (Me)Ph 5, Ph26, (p-MeC6H4)27) were obtained in low-to-moderate (12–26%) yields but reproducible yields in an unexpected metal-mediated reaction in MeOH between the nickel salt NiCl2·2H2O, N,N-disubstituted cyanamides NCNR2, and the amidoxime MeC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH)NH2. These complexes were formed along with a spectrum of cyanamide–oxime coupling products. The IR and X-ray data indicate the delocalization within the NNO and NCN systems of the nitrosoguanidinate ligand. This delocalization was additionally confirmed by inspection of Wiberg bond indices for the selected bonds. In the X-ray structure of 5, the rare metallophilic contacts Ni⋯Ni between stacks of the square-planar complexes were detected and these non-covalent interactions were studied by non-relativistic and relativistic DFT calculations and topological analysis of the electron density distribution within the framework of Bader's theory (QTAIM method). The estimated strength of the Ni⋯Ni interactions is 1.3–1.9 kcal mol−1 and they are mostly determined by crystal packing effects and weak attractive interactions between adjacent metal centers due to the overlapping of their dz2 and pz orbitals.
NOH)NH2. These complexes were formed along with a spectrum of cyanamide–oxime coupling products. The IR and X-ray data indicate the delocalization within the NNO and NCN systems of the nitrosoguanidinate ligand. This delocalization was additionally confirmed by inspection of Wiberg bond indices for the selected bonds. In the X-ray structure of 5, the rare metallophilic contacts Ni⋯Ni between stacks of the square-planar complexes were detected and these non-covalent interactions were studied by non-relativistic and relativistic DFT calculations and topological analysis of the electron density distribution within the framework of Bader's theory (QTAIM method). The estimated strength of the Ni⋯Ni interactions is 1.3–1.9 kcal mol−1 and they are mostly determined by crystal packing effects and weak attractive interactions between adjacent metal centers due to the overlapping of their dz2 and pz orbitals.


 Please wait while we load your content...
Please wait while we load your content...