Novel red-emitting phosphors A2HfF6:Mn4+ (A = Rb+, Cs+) for solid-state lighting†
Abstract
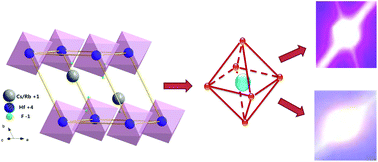
New red-emitting phosphors A2HfF6:Mn4+ (A = Rb+, Cs+) with a single phase have been successfully synthesized via a simple ion exchange method, and their structures and luminescence properties were investigated. It was found that Mn4+ ions in Rb2HfF6 and Cs2HfF6 who share wide band gaps can possess broad excitation bands in the blue regions and intense red emission with internal quantum yields of 0.556 and 0.652, respectively. Meanwhile, these red phosphors exhibit high chemical and thermal stabilities. A series of LED devices with a tunable color rendering index and color temperature were fabricated with these samples which can remarkably optimize the optical performances of white light-emitting diodes (w-LEDs). These results indicate that A2HfF6:Mn4+ phosphors can be promising red phosphors in w-LEDs.


 Please wait while we load your content...
Please wait while we load your content...