Mesoporous Mn2O3/reduced graphene oxide (rGO) composite with enhanced electrochemical performance for Li-ion battery†
Abstract
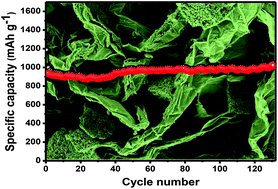
Transition metal oxides are the most promising candidates in low-cost and eco-friendly energy storage/conversion applications. Herein, bare Mn2O3 and a Mn2O3/reduced graphene oxide (rGO) composite have been synthesized by a facile chemical co-precipitation and subsequent annealing procedure. The synthesized Mn2O3/rGO composite exhibits a porous microcube structure formed with several interconnected particles. The porous Mn2O3/rGO composite, with high surface area and increased conductivity, facilited the charge transfer to enhance the overall electrochemical performance when applied as an anode material in Li-ion batteries. The porous Mn2O3/rGO composite exhibits a highly reversible lithium storage capacity of 1015 mA h g−1 at a rate of 0.5 C (230 mA g−1) during 130 cycles with excellent cycling stability and rate capability. The superior electrochemical performance results mainly due to the combined effect of rGO and Mn2O3, which offers high conductivity, faster Li+ ion transfer, and enhanced structural stability. The material synthesis strategy presented in this study is simple, cost-effective and scalable, which can open new avenues for large-scale applications of composites of graphene and other transition metal oxides.


 Please wait while we load your content...
Please wait while we load your content...