Alkali and alkaline earth metal salts of tetrazolone: structurally interesting and excellently thermostable†
Abstract
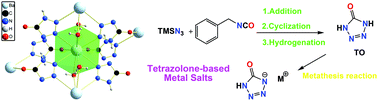
Tetrazolone (5-oxotetrazole) was synthesized by a moderate strategy through three steps (addition, cyclization and catalytic hydrogenation) avoiding the unstable intermediate diazonium, as reported during the previous preparation. Alkali and alkaline earth metal salts with lithium (1), sodium (2), potassium (3), rubidium (4) caesium (5), magnesium (6), calcium (7), strontium (8) and barium (9) were prepared and fully characterized using elemental analysis, IR and NMR spectroscopy, DSC and TG analysis. All metal salts were characterized via single-crystal X-ray diffraction. They crystallize in common space groups with high densities ranging from 1.479 (1) to 3.060 g cm−3 (5). Furthermore, the crystal structures of 7, 8 and 9 reveal interesting porous energetic coordination polymers with strong hydrogen bond interactions. All new salts have good thermal stabilities with decomposition temperature between 215.0 °C (4) and 328.2 °C (7), significantly higher than that of the reported nitrogen-rich salt neutral tetrazolone. The sensitivities towards impact and friction were tested using standard methods, and all the tetrazolone-based compounds investigated can be classified into insensitive. The flame test of these metal salts supports their potential use as perchlorate-free pyrotechnics or eco-friendly insensitive energetic materials.


 Please wait while we load your content...
Please wait while we load your content...