Impact of aryloxy initiators on the living and immortal polymerization of lactide†
Abstract
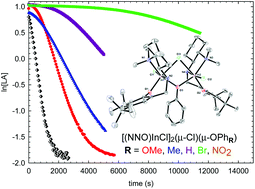
This report describes two different methodologies for the synthesis of aryl end-functionalized poly(lactide)s (PLAs) catalyzed by indium complexes. In the first method, a series of para-functionalized phenoxy-bridged dinuclear indium complexes [(NNO)InCl]2(μ-Cl)(μ-OPhR) (R = OMe (1), Me (2), H (3), Br (4), NO2 (5)) were synthesized and fully characterized. The solution and solid state structures of these complexes reflect the electronic differences between these initiators. The polymerization rates correlate with the electron donating ability of the phenoxy initiators: the para-nitro substituted complex 5 is essentially inactive. However, the para-methoxy variant, while less active than the ethoxy-bridged complex [(NNO)InCl]2(μ-Cl)(μ-OEt) (A), shows sufficient activity. Alternatively, aryl-capped PLAs were synthesized via immortal polymerization of PLA with A in the presence of a range of arylated chain transfer agents. Certain aromatic diols shut down polymerization by chelating one indium centre to form a stable metal complex. Immortal ROP was successful when using phenol, and 1,5-naphthalenediol. These polymers were analysed and chain end fidelity was confirmed using 1H NMR spectroscopy, MALDI-TOF mass spectrometry, and UV-Vis spectroscopy. This study shed light on possible speciation when attempting to generate PLA-lignin copolymers.


 Please wait while we load your content...
Please wait while we load your content...