A simple quinolone Schiff-base containing CHEF based fluorescence ‘turn-on’ chemosensor for distinguishing Zn2+ and Hg2+ with high sensitivity, selectivity and reversibility†
Abstract
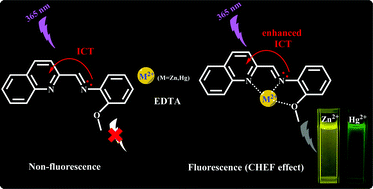
A new simple ‘dual’ chemosensor MQA ((E)-2-methoxy-N-((quinolin-2-yl)methylene)aniline) for distinguishing Zn2+ and Hg2+ has been designed, synthesized and characterized. The sensor showed excellent selectivity and sensitivity with a fluorescence enhancement to Zn2+/Hg2+ over other commonly coexisting cations (such as Na+, Mg2+, Al3+, K+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Ga3+, Cd2+, In3+ and Pb2+) in DMSO–H2O solution (1/99 v/v), which was reversible with the addition of ethylenediaminetetraacetic acid (EDTA). The detection limit for Zn2+/Hg2+ by MQA both reached the 10−8 M level. The 1 : 1 ligand-to-metal coordination patterns of the MQA-Zn2+ and MQA-Hg2+ were calculated through a Job's plot and ESI-MS spectra, and were further confirmed by X-ray crystal structures of complexes MQA-Zn2+ and MQA-Hg2+. This chemosensor can recognize similar metal ions by coherently utilizing intramolecular charge transfer (ICT) and different electronic affinities of various metal ions. DFT calculations have revealed that the energy gap between the HOMO and LUMO of MQA has decreased upon coordination with Zn(II)/Hg(II).


 Please wait while we load your content...
Please wait while we load your content...