Unveiling the effects of the in situ generated arene anion radical and imine radical on catecholase like activity: a DFT supported experimental investigation†
Abstract
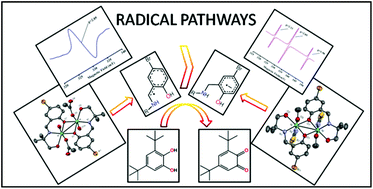
Two new dinuclear nickel(II) complexes namely [Ni2(L1)2(OAc)2(H2O)2]·CH3CN (1) and [Ni2(L2)2(SCN)2(CH3OH)2]·CH3OH (2) have been synthesized from the designed Schiff-base ligand 4-bromo-2-[(2-hydroxy-1,1-dimethyl-ethylimino)-methyl]-phenol (HL1) and its reduced analogue 4-bromo-2-[(2-hydroxy-1,1-dimethyl-ethylamino)-methyl]-phenol (HL2), respectively. Both 1 and 2 have been characterised by usual physicochemical techniques (UV-Vis, FT-IR, ESI-MS study and single crystal XRD) and their variable temperature magnetic study has been performed. The nickel(II) centres in the dinuclear complexes 1 and 2 are antiferromagnetically coupled through participation of the bridging phenoxyl oxygen. In acetonitrile solution both 1 and 2 retain their dinuclear structural integrity as is evident from the ESI-MS study. The catecholase-like activity of 1 and 2 has been performed in acetonitrile medium using 3,5-di-tert-butylcatechol (3,5-DTBC) as a model substrate. Complex 1 shows a higher catalytic activity than that of complex 2. The ESI-MS study suggests that dinuclear species undergo cleaving into a mononuclear entity in the presence of 3,5-DTBC and that mononuclear species are supposed to act as active catalysts in the catalytic cycle. The EPR study of catalytic reactions confirms that organic radicals have been generated during catalysis in both cases. However, in the case of complex 1 catalyzed reaction a single isotropic signal at g = 1.97 is obtained which is most likely due to imine radical formation. On the other hand, for complex 2 catalyzed reaction the spectrum shows a signal with hyperfine splitting (g = 2.11, 2.05 and 1.9), thereby suggesting the generation of a new radical i.e. an arene anion radical in this study on catecholase activity. Extensive DFT calculations have been performed to support the experimental observation and thus to put forward the most probable mechanistic pathways operating in the two cases. The higher efficiency of the imine radical pathway over the arene anion radical has been rationalized by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...