Effect of the apical ligand on the geometry and magnetic properties of copper(ii)/mesoxalate trinuclear units†
Abstract
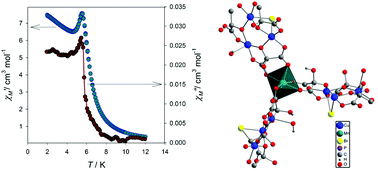
Three new heterometallic metal–organic frameworks, namely, {(Ph4P)2[MnCu3(Hmesox)3Br(H2O)]·H2O}n (1), {(Ph4P)2[CoCu3(Hmesox)3Br]}n (2) and {(Ph4P)2[ZnCu3(Hmesox)3Br]·2.5H2O}n (3) were prepared and their structure and magnetic properties were investigated (H4mesox = mesoxalic acid, Ph4P+ = tetraphenylphosphonium). The structure of all the compounds consist of two interpenetrating opposite-chirality supramolecular cationic and polymeric anionic 3-D (10,3)-a networks, which results in chiral compounds. The anionic network is formed from the polymerization of [Cu3(Hmesox)3Br]4− units, working as three connectors, and M(II) cations, working as three-connecting nodes, M = Mn(II), Co(II) and Zn(II). The Ph4P+ cations build the cationic chiral supramolecular network opposite to the anionic one. Compounds 1 and 2 exhibit long-range magnetic ordering with critical temperatures of 7.2 K and 6.9 K, respectively. However, compound 3 does not display long-range order, but shows ferromagnetic and antiferromagnetic coupling among the Cu(II) ions. The magnetic interactions are studied by DFT calculations and compared with related Cu(II)-mesoxalate compounds previously reported.


 Please wait while we load your content...
Please wait while we load your content...