Structural stability and electronic properties of an octagonal allotrope of two dimensional boron nitride
Abstract
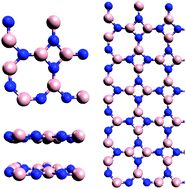
An octagonal allotrope of two dimensional boron nitride is explored through first principles calculations. Calculations show that two dimensional octagonal boron nitride can be formed with a binding energy comparable to two dimensional hexagonal boron nitride. In addition, two dimensional octagonal boron nitride is found to have a band gap smaller than two dimensional hexagonal boron nitride, suggesting the possibility of semiconductive attributes. Two dimensional octagonal boron nitride also has the ability to layer through physisorption. Defects present within two dimensional octagonal boron nitride also lead toward the introduction of a magnetic moment through the absence of boron atoms. The presence of defects is also found to render both hexagonal and octagonal boron nitrides reactive against hydrogen, where greater reactivity is seen in the presence of nitrogen. Thus, two dimensional octagonal boron nitride is confirmed with potential to tailor properties and reactivity through lattice shape and purposeful introduction of defects.


 Please wait while we load your content...
Please wait while we load your content...