Dibenzothiophene-platinated complexes: probing the effect of ancillary ligands on the photophysical performance†
Abstract
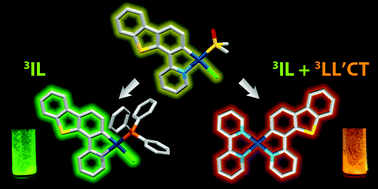
Cyclometalation of dibenzothienyl-pyridine (HPyDBT) afforded a series of platinum(II) complexes Pt(PyDBT)(L)Cl (L = DMSO, 1; P(p-C6H4-X)3 (X = H, 2; CF3, 3; OMe, 4; NPh2, 5); 1,3,5-triaza-7-phosphaadamantane, 6; 2,6-dimethylphenyl isocyanide, 7). Chelating bidentate LL ligands formed cationic compounds [Pt(PyDBT)(LL)]+ (LL = 1,2-bis(diphenylphosphino)benzene, 8; 2,2′-bipyridine, 9; 1,10-phenanthroline, 10). Oxidation of a thienyl sulfur atom allowed for the isolation of the sulfone derivative Pt(PyDBT)(PPh3)Cl (11). The title complexes were characterized crystallographically (except 7). Investigation of their photophysical behavior revealed solid state phosphorescence with quantum yields up to 0.45 for neat powders. The ancillary ligands L show a minor influence on the emission energies of the neutral compounds, but affect dramatically the intensity of luminescence. In contrast, the cationic species with diimine ligands demonstrate a significant contribution of the LL fragments into the emissive T1 states that leads to a certain mixing of 3IL and 3LL′CT transitions and causes a substantial bathochromic shift of emission.


 Please wait while we load your content...
Please wait while we load your content...