Iron–cobalt bimetal oxide nanorods as efficient and robust water oxidation catalysts†
Abstract
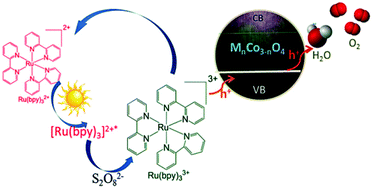
Cobalt-based oxides are considered as potential water oxidation catalysts for future artificial photosynthetic systems because of their high abundance, strong stability and efficient performance. Herein, a series of cobalt-based oxides, MnCo3−nO4 (M = Mn, Fe, Co) samples, were synthesized through changing the metal sources by a low-temperature coprecipitation method. These catalysts were investigated under photochemical and electrochemical water oxidation conditions. And they all exhibited efficient activity for water oxidation under alkaline, acidic and neutral conditions under visible light irradiation. An excellent O2 yield of 90.4% for Fe–Co bimetal oxide (Fe1.1Co1.9O4) nanorods was obtained under optimal conditions (photoirradiation at λ ≥ 420 nm, [Ru(bpy)3](ClO4)2 as the photosensitizer, Na2S2O8 as the oxidant in borate buffer at pH = 9.0, bpy = 2,2-bipyridine). Among MnCo3−nO4 samples, Fe1.1Co1.9O4 nanorods were proved to be the optimal electrocatalytic water oxidation catalyst as well. Multiple experiments (SEM, FT-IR, XRD, XPS, Bulk electrolysis) were used to test the stability of Fe1.1CO1.9O4 and these results indicate that Fe1.1CO1.9O4 nanorods are highly stable. Furthermore, based on Mott–Schottky and cyclic voltammetry analysis, the best balanced flat-band potential of Fe1.1CO1.9O4 nanorods is just located at the middle position between the oxidation potential of O2/H2O and the half-wave potential of [Ru(bpy)3]3+/2+, which was probably responsible for their superior photocatalytic water oxidation performance.
- This article is part of the themed collection: The Role of Inorganic Materials in Renewable Energy Applications


 Please wait while we load your content...
Please wait while we load your content...